Recently I received a phone call from a no-till farmer in east-central Nebraska asking why his corn looked yellow even though he thought his nitrogen (N) program was adequate. After asking a few questions, I drew the conclusion that the fertility problem was a sulfur (S) deficiency.
Sulfur deficiency, like N, is expressed as slow growth and general plant yellowing, often with thinner stems and a spindly appearance. The yellowing (chlorosis) starts with interveinal yellowing of new leaves and develops gradually over the entire leaf area. Yellowing of leaves occurs first on newer growth and extends to the older leaves in some crops. With time, reddening and purpling develops in many crops, especially in species of the Brassicaceae (Mustard) Family. Movement of S within the plant is not as great as that of N, so S deficiency does not cause ‘firing’ of the lower leaves as does N deficiency.
In many of our field crops, it is difficult to visually distinguish between N and S deficiency, although plant tissue testing can differentiate these with greater certainty.

Sulfur-deficient wheat is pale green to almost yellowish, in stark contrast to the darker healthier areas in this field of second-year wheat. Sulfur deficiency often becomes quite obvious by late jointing or boot stage, although it is more difficult to pick out earlier in the plant’s life cycle.
Sulfur in Soils
Sulfur occurs in soils in organic and inorganic forms, which are the categories used by chemists to refer to whether carbon (C) is contained in the molecule. In most soils the greatest reserve of S is in the organic form. Organic S means it is bound to the organic matter. In a previous article (March ’03 Leading Edge), I wrote about the C:N ratio. Actually, most plant nutrients are held to some extent in the organic matter, and sulfur occurs in soil organic matter (OM) at approximately 1/8 the rate of N. So in general, the C:N:S ratio in soils is about 100:8:1. Since soil organic matter is about 58% carbon, the OM:N:S ratio is 170:8:1. This means that for every 170 pounds of soil OM there is 1 pound of S. This organic S becomes available to plants through microbial activity called mineralization. In the process of mineralization, H2S (hydrogen sulfide) is formed, which under aerobic (oxygen-available) conditions is readily converted to sulfate (SO4) ions. Sulfate is the form of S taken up by plants.
Sulfate is a soluble anion (negatively charged ion), just like nitrate, that can leach with soil water and be lost below the root zone. In more arid areas, sulfate may accumulate within the root zone since leaching potential is less. In some cases where the root zone has water-movement restrictions, sulfate is one of the anions that combines with the (positively charged) cations sodium (Na), calcium (Ca), or magnesium (Mg) to form salts which can accumulate to high enough levels to create soil salinity. However, salinity is relatively rare in most of the soils of the central U.S. Plains.
Since most of the soil S is held in the organic matter, it is obvious that crops grown on soils with very low OM levels would be the most vulnerable to S deficiencies. It is well known that sandy soils have shown S deficiencies for many years. Eroded soils that have lost a lot of topsoil that was high in organic matter also show S deficiencies frequently. Now we are beginning to see S deficiencies in no-till fields where soil OM levels are relatively good. What is causing the problem?
One factor is cleaner air. The atmosphere is one of the S sources for plants. Atmospheric sulfur dioxide (SO2) combines with water to form sulfuric acid (H2SO4) in rainfall. As industry has reduced SO2 emissions from factories, less S is being supplied by rainfall in some regions. However, considerable S occurs naturally in rainfall, since the majority of atmospheric S is of natural origins such as emissions from volcanoes and geologic sulfur vents, and volatile S compounds arising from soil OM decay and biological processes. On the U.S. Plains, in areas distanced from industrial or other significant manmade SO2 emissions, rainfall contributes 5 or 6 lbs/a/yr of S on average, although large variations occur annually.

S-deficient wheat following soybeans.
Another source of S for crops is from the application of P fertilizers, since sulfuric acid is used to make phosphate fertilizers. In the past, superphosphate (single) was commonly applied, which contained about 12% S, while MAP or DAP often contained 2% S. However, the fertilizer industry has been increasing the purity of their phosphate fertilizers, thereby decreasing the amount of S in those fertilizers.
In many regions, a substantial amount of S has historically been supplied to crops by the depletion of organic matter in our soils by doing tillage. With no-till farming, less organic matter is mineralized each season, especially in the early stages of no-till adoption while surface residue levels are accumulating and as soil OM levels begin to slowly increase because soil stirring is eliminated. In the process of increasing organic matter, mineralization of organic S to sulfate has slowed. Less sulfate is released for crop uptake. The combination of less mineralization, intensified cropping, less S from the air, and more pure fertilizers has increased the likelihood for S deficiencies to appear in our no-till crops.
As no-tillers try to build organic matter, one must remember that in addition to sequestering carbon and N, sulfur must also be sequestered. Every 170 pounds of organic matter gained by the soil will contain 8 pounds of N and 1 pound of S. One percent organic matter in 8 inches of soil amounts
to 24,000 pounds per acre. This quantity of OM holds over 1,100 lbs of N per acre and 140 lbs of S per acre. If soil OM is increasing instead of being depleted, this is a net sink rather than a source for sulfur, so the potential for more S deficiencies in the future is great without S fertilization.
Another source of S for plants is sulfate in irrigation water. If you irrigate on your farm, test the water for sulfate: a concentration greater than 8 – 10 ppm SO4-S is adequate for crop requirements. In many areas, sulfate in irrigation water is great enough that no additional S needs to be supplied to the crop.
Sulfur in Plants
Most of the S in plants can be found in the broad groups of 1) proteins and peptides, 2) volatile organic compounds that give onions and some other crops their characteristic odors, 3) mustard oil glucosides, and 4) inorganic sulfate ion (SO4). Proteins and peptides account for 90% of the S found in the plant. Oils of some plants, especially from the Mustard and Onion Families, are rich in S. Sulfur fertilization has been shown to increase oil concentration in flax and soybean grain.
Sulfur is a constituent of the amino acids cystine, cysteine, and methionine. These amino acids are essential in protein formation within the plant. S also activates certain proteolytic enzymes and is a component of coenzyme A, glutathione, and certain vitamins. Sulfur is found in many other compounds in the plant with unknown functions. Since S and N are both necessary for building proteins in plants, a strong relationship exists between N and S in crop nutrition.
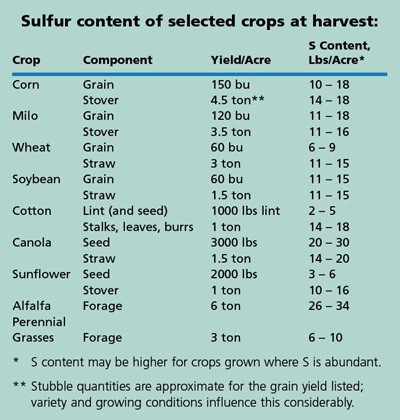
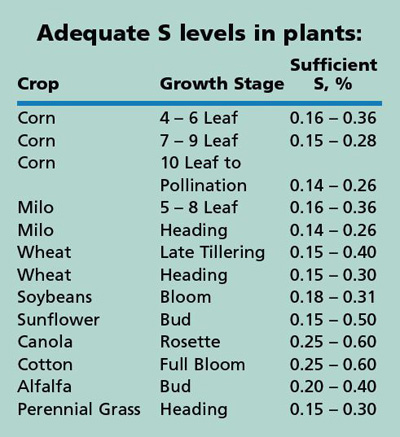
As shown in the tables, sulfur acquisition and needs differ greatly among plant species, among varieties within a species, and with stage of development of the crop. Plant S concentrations can also vary widely depending on the amount of S available. Grass crops have the lowest S concentration in their grain, legumes have about twice the S concentration of grasses, and Brassicaceae (mustards, canola, etc.) have three times the concentration of S. The total sulfur requirement of grass crops is generally 1/7 to 1/12 of the N requirement.

Sulfur-deficient wheat up close. Note the paleness extends throughout the plant, although slightly more pronounced on the newest leaves. Overall, the plants are more spindly than normal. Severe sulfur deficiency results in very little tillering.
Sulfur Availability
As previously described, crops most likely to show S deficiencies are on soils with low organic matter or where soil OM is mineralizing slowly. However, soils located near industrial sites may receive enough S from the atmosphere to supply crop needs. How can we estimate how much S the soil has available for crop uptake?
Numerous soil test methods exist for evaluating plant-available sulfur. In the North Central States Region, the calcium-phosphate extract seems to predict available sulfate (SO4) more consistently than other tests. In the surface 0 – 8 inches of soil, a sulfate test reading of 6 ppm SO4-S or less is considered low with the likelihood of sulfur-deficient crops occurring unless additional sulfur is supplied. For values of 6 – 10 ppm SO4-S, the sulfate test is in the medium range and a deficiency may be exhibited at times. A soil sulfate test above 10 ppm has generally been considered adequate for most cropping systems, although some experiences with no-till may yet cause this to be reevaluated.
In some cases it is an advantage to sample the subsoil from 8 to 24 inches to measure sulfate in more of the root zone. If the subsoil test is also less than 6 ppm, the possibility of an S deficiency is greater. If the subsoil value is more than 10 ppm SO4-S, the chance of an S deficiency is reduced considerably, although occasional deficiency symptoms may still occur especially in small areas of the field.
Sulfur Fertilization
There are several commercial S fertilizers (see table). Common sulfur fertilizers with high solubility are ammonium sulfate, 21-0-0-24, and ammonium thiosulfate, 12-0-0-26. The sulfur in ammonium sulfate is already in the plant-available form, SO4. It is a dry fertilizer and can be blended with other dry fertilizers, or made into liquid 8-0-0-9. Ammonium thiosulfate is a higher analysis liquid that reacts in the soil to create some SO4 (immediately available) as well as some S in a form that must be oxidized to SO4 before it is available to plants; this oxidation occurs in the presence of soil microorganisms. Ammonium thiosulfate can be blended with other liquids, although 12-0-0-26 should not be applied in the seed furrow since small amounts are toxic to germinating seeds.
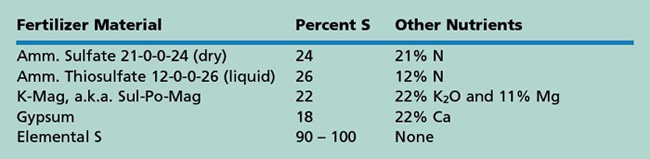
Potassium magnesium sulfate (K-Mag or Sul-Po-Mag) is a dry fertilizer that also has the S in the SO4 form. It is a source for potassium (K) and magnesium (Mg) as well, and can be safely applied in the seed furrow within limits for K2O for that crop and row spacing. Gypsum (CaSO4), another source of S, is a dry product often used as a soil amendment.
Elemental sulfur fertilizer is in a form that must be oxidized to the SO4 ion for plant uptake. The oxidation occurs by microorganism activity. The population of these microbes in the soil is low so it takes time for the elemental S to become available, particularly if temperatures are cool or moisture is too low for the microbes to be active. It may take several months to a year for all the elemental S to be converted to sulfate. If an immediate response to S is needed, elemental S is not adequate. If a supply of S is needed for a long period such as for alfalfa or perennial grass, then this source can be used. Response will be slow and will continue for a longer period of time than the readily available forms of sulfur. Elemental S can be used for annual crops if applied every year so that a nearly continuous supply of sulfate is being produced.

This corn plant is sulfur deficient. N- and S-deficiency symptoms sometimes appear rather similar.
Elemental sulfur is sometimes used to lower the pH of very alkaline soils. As the elemental S gets converted to SO4 by microbial action, hydrogen (H) ions are released which is an acidifying reaction (by definition). There is a very slight drop in soil pH on the average, but around the S granules the pH is reduced enough that crop color and production improve due to enhanced availability of other plant nutrients. Experience has shown that 300 – 500 pounds of elemental S per acre will benefit crops growing on highly alkaline soils containing excess lime.1 On acid soils, elemental S will cause more soil acidity; this high rate of elemental S is only for high pH soils with excess lime.
Because sulfur is crucial for building plant protein, it is needed in the plant at the same time N is needed. A good practice is to include S fertilizer with the N application. It is easy to mix ammonium thiosulfate with 28% or 32% N and apply these products at the same time, or to blend dry S sources with urea. However, if these materials are applied while the crop is growing (top-dress or side-dress), it is important to ensure the crop has access to adequate S at early development stages, which may require supplying some S closer to the time of planting.
Sulfur Recommendations
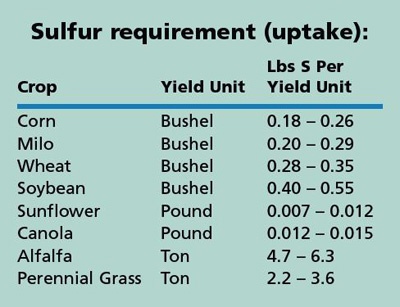
The amount of S fertilizer needed depends on the SO4-S level in the soil, OM level, crop species, and on the yield potential of the crop. The S requirement of several crops is shown in the table (crop requirement is total uptake of S for normal development of roots, stems, leaves, and seeds). The S requirement is multiplied by the yield goal or yield potential to arrive at the total amount of S needed by the crop, whether it is supplied by soil, rainfall, or fertilizer application.
The SO4-S test previously described can measure the soil’s ability to supply S to the crop. To estimate the S availability of your soil, multiply the SO4-S test result by 0.15, then multiply by the sampling depth in inches (6 or 8 inches). Subtract this value from the S requirement of the crop at the yield goal to determine how much (if any) S should be added.
An example for wheat: The yield goal is 70 bu/a and the sulfate soil test is 5 ppm S. The crop S requirement would be between 20 and 25 lbs/a (70 x 0.28, or 70 x 0.35). The soil S availability value is 6 lbs/a (5 ppm x 0.15 x 8 inches). The difference is 14 to 19 lbs/a of S fertilizer that would need to be supplied to have a good probability of having adequate sulfur nutrition to reach the yield potential of the crop.2
Sulfate is a highly soluble anion and will move with soil water much as nitrate does. Therefore S fertilizer should be applied as part of an annual program if you are in a deficit area. In general, one should not try to build the SO4-S soil test level because the carryover SO4-S will eventually move deeper than the root zone by leaching.
Determining Sulfur Deficiency
We can use a sulfur soil test and organic matter level to give us a good idea of whether to expect a sulfur deficiency. However, if the crop is growing, how can we check the sulfur status of the crop? Samples can be collected of aboveground portions of plants and analyzed for the suspected deficiency (most agricultural laboratories do plant tissue analyses). Requesting analysis for a majority of the nutrients will provide the best gauge of plant nutrition. If areas of the field are growing poorly or exhibit unusual leaf-coloration patterns, it is best to sample plants from these areas as well as separately sampling plants from better areas to provide a benchmark or comparison. As mentioned before, nitrogen and sulfur
deficiencies sometimes appear rather similar; therefore it is important to have both nutrients analyzed.
The table shows the adequate (sufficiency) levels of S in the plant at a given growth stage. Another guideline for interpretation of plant analyses is the N:S ratio. For grass crops, N:S greater than 16:1 is a strong indicator that S is inadequate. For canola, turnips, and other Brassicaceae, N:S of 9:1 or greater indicates a likely S deficiency.

This field of wheat following soybeans was acutely sulfur deficient. A late-season freeze defoliated most of the field, adding insult to injury. However, a few patches of healthy wheat (adequate nutrition) had sufficient canopy to escape freezing, which are the tall green clumps. Sulfur deficiencies are becoming more common, especially with no-till.
If a deficiency is diagnosed early in the crop’s growth, S fertilizer can be applied. Sulfate is soluble and can be taken up as soon as it is added and moisture moves it into the root zone. This is similar to nitrogen.
Summary
Sulfur deficiency in crops is increasing, especially in no-till. Growers must be alert to the potential for sulfur deficiency and be informed about sulfur fertilization. The amount of S needed to correct a deficiency is fairly small compared to nitrogen and phosphorus, although S is equally as essential as N or P for normal crop growth and good yields. Usually an application of 10 – 20 lbs/a of S will prevent or correct the deficiency for the crop year, and at very low cost. Since S becomes highly susceptible to leaching losses once it converts to sulfate, S fertilization should be considered a necessary annual application instead of trying to build the level in the soil. Producers should continue to monitor their crops for pale green to yellowish areas that could be caused by sulfur deficiencies, and take tissue samples of suspect areas. Failing to supply adequate S will impair soil organic matter accumulation and can cause substantial crop yield losses.
1 Many other sulfur-containing materials can also benefit crops growing on soils with excess lime, due to acidifying reactions and the elimination of some of the bicarbonate in solution near the root zone. Bicarbonate hinders plant utilization of many micronutrients.
2 Laboratories and scientists have various equations for making S recommendations, some of which use certain baseline requirements (e.g., a ‘universal’ value of 40 lbs/a of S needed by the crop, minus soil test levels to 6- or 24-inch depths), while others will take into account the soil OM reading and essentially predict that a certain quantity of S mineralization will occur. Due to economics, sulfur recommendations have not received the extensive research and scrutiny that N guidelines have undergone, and there is little consensus yet in the industry as to the best method for calculating sulfur needs.
Author: Raymond C. Ward, a soil scientist & founder of Ward Science Laboratories at Kearney, NE.